Abstract
Introduction: InO, a humanized anti-CD22 antibody conjugated to calicheamicin, produced superior response rates vs SC for R/R ALL in INO-VATE (complete remission rates [CR]/CR with incomplete hematologic recovery [CRi], 80.7% [95% CI, 72.1-87.7] vs 29.4% [21.0-38.8]; 2-sided P <0.001; Kantarjian H. NEJM 2016). Here we report the long-term efficacy and safety of InO vs SC (intensive chemotherapy) in patients with R/R ALL.
Methods: In INO-VATE (NCT01564784), adults with CD22-positive ALL due to receive salvage 1 or salvage 2 treatment were randomized 1:1 to InO (n=164; starting dose 1.8 mg/m2/cycle [0.8 mg/m2 on d 1; 0.5 mg/m2 on d 8 and 15 of a 21-28 d cycle for ≤6 cycles]) or SC (n=162; either fludarabine/high-dose (HD) cytarabine/granulocyte colony-stimulating factor, HD Ara-C plus mitoxantrone, or HD Ara-C). Outcomes presented herein include overall survival (OS), progression-free survival (PFS), predictors of OS determined by stepwise Cox regression modeling, and safety. Following treatment discontinuation, patients were followed for survival for up to 5 years or 2 years from randomization of the last patient, whichever occurred first.
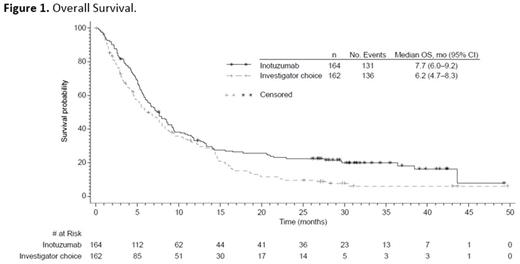
Results: As of January 5, 2017, median OS follow-up for all randomized patients was 6.6 (range, 0.0‒49.7) mo. OS was longer for InO vs SC with an estimated hazard ratio (HR; InO vs SC) of 0.751 (97.5% CI, 0.568-0.993) and a 1-sided stratified log-rank P=0.0105, indicating a 24.9% reduction in the risk of death in favor of InO (Figure 1). Median OS was 7.7 (95% CI, 6.0-9.2) vs 6.2 (4.7-8.3) mo in InO vs SC pts. At 12 mo, the estimated probability of OS was 33.6% (95% CI, 26.4-40.9) vs 32.0% (24.7-39.6) in InO vs SC patients, respectively, and 22.8% (16.7-29.6) vs 10.0% (5.7-15.5) at 24 mo. InO demonstrated significant improvement in PFS vs SC (HR, 0.450 [97.5% CI, 0.336-0.602]; 1-sided stratified log-rank P ˂0.0001; median PFS, 5.0 [95% CI, 3.9-5.8] vs 1.7 [1.4-2.1] mo). At 12 mo, the estimated probability of PFS was 18.2% (95% CI, 12.3-24.9) vs 4.9% (2.0-9.8) in InO vs SC patients, respectively and 13.2% (8.0-19.8) vs not estimable at 24 mo. In multivariate analysis, longer duration of first remission (continuous; P=0.0157), receiving follow-up hematopoietic stem cell transplant (HSCT; P ˂0.0001), minimal residual disease negativity (best status; P=0.0085), and attaining CR/CRi ( P=0.0214) were associated with lower risk of death in InO patients. In the safety population comprising patients who received ≥1 dose of study drug, 79 (48.2%) InO patients proceeded to HSCT with or without intervening induction or salvage therapy vs 35 (24.5%) SC patients. The most frequent grade ≥3 adverse events were hematologic, primarily cytopenias (InO, 79.9%; SC, 86.7%). Five veno-occlusive disease (VOD) cases (2 in patients with prior HSCT) occurred in InO pts and no VOD occurred in SC patients during treatment or in follow-up without intervening HSCT. Of the 79 InO patients who proceeded to HSCT, 18 (22.8%) experienced VOD (5 in patients with prior HSCT). Of the 35 SC patients who proceeded to HSCT, 3 (8.6%) experienced VOD (1 with prior HSCT).
Conclusions: Long-term follow-up in INO-VATE remained consistent with earlier reports showing that compared with SC, InO produced longer OS, prolonged PFS, and was an effective bridge to HSCT in patients with R/R ALL. Differences in OS were more pronounced at later time points, and differences in PFS were sustained at later time points. The toxicity profile of InO was consistent with previous reports and no new safety concerns were identified.
Kantarjian: Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding. DeAngelo: Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Immunogen: Honoraria, Research Funding; Blueprint Medicines: Honoraria, Research Funding; Incyte: Consultancy, Honoraria; BMS: Consultancy; Glycomimetics: Research Funding; ARIAD: Consultancy, Research Funding; Shire: Honoraria; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding. Liedtke: Prothena: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding; Celgene: Research Funding; Gilead: Research Funding; Bluebird bio: Research Funding. Stock: Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy. O'Brien: Aptose Biosciences, Inc.: Consultancy; Sunesis: Consultancy; AbbVie: Consultancy; Vaniam Group LLC: Consultancy; Celgene: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; ProNAI: Other: Research Support: Honorarium, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Astellas: Consultancy; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Alexion: Consultancy; GSK: Consultancy; Acerta: Other: Research Support: Honorarium, Research Funding; Janssen: Consultancy; Amgen: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Pfizer: Consultancy, Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Wang: Pfizer: Employment, Equity Ownership. White: Pfizer: Employment, Equity Ownership. Sleight: Pfizer: Employment, Equity Ownership. Vandendries: Pfizer: Employment, Equity Ownership. Advani: Pfizer: Consultancy; Takeda/ Millenium: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal